 Caltech institutions’s newly invented ‘Artificial leaf‘ is a highly efficient stand-alone photoelectrochemical (PEC) device which can split water into hydrogen and oxygen using sun light.
Caltech institutions’s newly invented ‘Artificial leaf‘ is a highly efficient stand-alone photoelectrochemical (PEC) device which can split water into hydrogen and oxygen using sun light.
“The first car driven by a child born today could be powered by hydrogen and pollution free,” ex-President George W. Bush said in his 2003 State of the Union address to the scientists and engineers about the Fuel Cell Vehicles (FCVs). He allocated $1.2 billion for the hydrogen research.
Later, in May 2009, under the advice of the then first secretary of Energy of President Obama, Dr. Steven Chu, the Nobel Prize-winning physicist, who said: “We asked ourselves, ‘Is it likely in the next 10 or 15, 20 years that we will convert to a hydrogen car economy?’. The answer, we felt, was ‘no.’”, this appropriation of funds was eliminated by the Obama administration, whose attention shifted towards the battery-powered vehicles.
Hydrogen is a not an energy source, but only a way of storing and tranporting energy. Though the electric vehicles powered by the hydrogen fuel cells (FCEVs) do not emit carbon dioxide, they should be categorized as a carbon-negative mode of transport, if the hydrogen used by them comes from carbon-negative sources like coal and natural gas.
Currently, the majority portion of the Hydrogen produced, almost 95%, is from fossil fuels – by steam reforming or partial oxidation of natural gas or coal gasification – either way, producing carbon dioxide as a by-product. There are also other sources like solar, biomass, wind and geothermal, which are renewable, and nuclear and oil, which are non-renewable. Last week, Toyota has launched an effort to test a renewable and fully carbon-neutral hydrogen supply chain powered by wind energy in Japan. Coming to storing solar power in the form of hydrogen, while there are various methods like solar pv and solar thermal, ‘Artificial Leaf’ is one among them.
Hydrogen is the simplest and the most abundant element in the universe. Though it is not available in gaseous form on earth, all we have to do is crack water and get hydrogen and oxygen. Though there are many methods to do this splitting job, artificial leaf does that in an environment-friendly way, using just solar energy.
Many scientists have been conducting researches on the Artificial leaf fantasy for the past three decades. While many of those theories looked plausible, only some entered the pre-commercial echelon. Caltech scientists are saying that they are one among those few.
 Caltech’s Joint Center for Artificial Photosynthesis (JCAP), for the last 5 years, has been working in this field to bring useful fuels out of sunlight, water, and Carbon dioxide by imitating the natural photosynthesis. Recently, they have published a paper in Energy and Environmental Science on their latest design.
Caltech’s Joint Center for Artificial Photosynthesis (JCAP), for the last 5 years, has been working in this field to bring useful fuels out of sunlight, water, and Carbon dioxide by imitating the natural photosynthesis. Recently, they have published a paper in Energy and Environmental Science on their latest design.
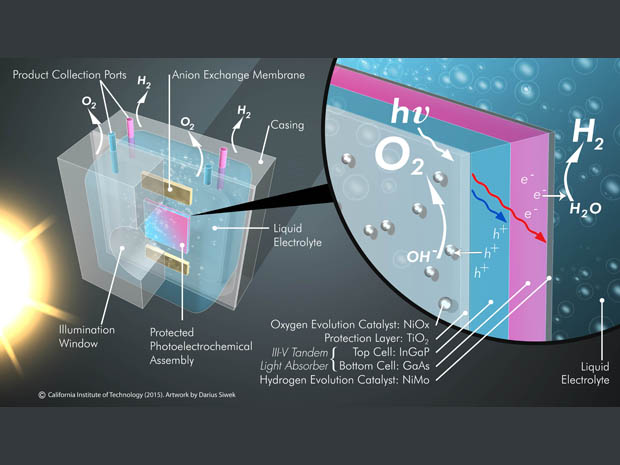
Caltech’s design employs a gallium arsenide photoanode and a photocathode separated by a semi-permeable membrane. Gallium arsenide is an amazing light absorber but, tends to oxidize in the presence of water. To protect and keep the photoanode stable, a layer of titanium dioxide is used. Another layer, which is of nickel, is applied on top of that to work as a catalyst.
On exposure to sunlight, the gallium arsenide photoanode oxidises water molecules and generates oxygen (O2), protons and electrons, which pass through the semi-permeable membrane, and form H2 upon recombination, which happens due to the presence of the photocathode.
Thus, the fully integrated stand-alone system developed by the Caltech researchers intakes water and separates hydrogen from oxygen in the presence of sunlight. Their achievement, in their own words, “shatters all of the combined safety, performance, and stability records for artificial leaf technology by factors of 5 to 10 or more.”
The device shown in the video (at the bottom) is a 1 Sq cm prototype operated for forty hours with above 10% efficiency, producing about 0.8 ml hydrogen per second. The scientists are still trying to perfect the device as they found that it is prone to degradation when left running indefinitely.
They found that the solar-to-hydrogen conversion efficiency, which was 11% initially, is gradually decreasing with time – to 10% after 40 hours, 9% after a total of 80 hours of operation. But, the happy news is that the scientists have found not only the cause of the degradation but also a possible solution for it. The further results will be posted as soon as they have gotten the solution to work. The team of scientists is pretty sure that the problem is solvable and let us hope for good results as Caltech is trying hard to take us one step closer to the dream that is Hydrogen economy.
Image(c): Caltech

































